| Numéro |
Radioprotection
Volume 53, Numéro 2, April-June 2018
|
|
|---|---|---|
| Page(s) | 107 - 113 | |
| DOI | https://doi.org/10.1051/radiopro/2018009 | |
| Publié en ligne | 12 avril 2018 | |
Article
Radiation dose from 18F-FDG PET/CT procedures: influence of specific CT model and protocols
School of Chemistry & Physics, University of KwaZulu-Natal, Pietermaritzburg,
Private Bag X01,
Scottsville
3209, South Africa
* Corresponding author: chettyn3@ukzn.ac.za
Received:
18
July
2017
Accepted:
24
February
2018
The increasing use of the integrated 18F-fluoro-deoxy-glucose (FDG) positron emission tomography/computed tomography (PET/CT) imaging modality in the management of tubercular lesions raises concerns about associated radiation exposure. This work aimed to study the effects of CT model and study protocols on the overall radiation dose from a PET/CT examination. Two PET/CT systems with five representative CT exposure protocols applied for clinical patients in PET/CT imaging following retrospective evaluation were studied. CT doses were calculated using the CT-Expo dosimetry software (version 2.4), while the PET component dose was estimated applying the International Commission on Radiological Protection (ICRP) 106 dose coefficients. The total effective dose ranged from 8.0 to 24.05 mSv for system I and 8.35 to 26.85 mSv for system II, resulting in differences of 4.3 to 15% for the low-dose scan and 4.1 to 11% for standard dose scans. The CT component contribution to the total dose was between 32 and 77% for system I, and 35 and 79% for system II. However, the contributions were not significantly different (p > 0.05) for all protocols. The observed variation in CT contribution represents a requisite pedestal on the need for a nation-wide dose assessment for further optimization of the imaging procedure to maximize benefit to patients.
Key words: positron emission tomography / computed tomography imaging / computed tomography scan / radiation exposure / effective dose
© EDP Sciences 2018
1 Introduction
The effectiveness of positron emission tomography with 18F-fluoro-deoxy-glucose (18F-FDG) in detecting active tuberculoma and other tuberculosis (TB)-related lesions, assessing the involvement of pulmonary and extra-pulmonary TB and its activity within the body, is well documented (Goo et al., 2000; Kim et al., 2008; Hahm et al., 2010; Skoura et al., 2015). The major concern of this non-invasive method of imaging is the additional radiation exposure from CT acquisition together with the internal exposure (ɣ-ray) from the administered tracer, since FDG-PET scans often require an anatomic imaging study typically a CT examination, for attenuation correction and optimal tracer uptake interpretation (Alessio et al., 2004; Karam et al., 2008; Soussan et al., 2012).
It is known that an 18F-FDG PET/CT scan is accompanied by increased radiation dose capable of enhancing the risk of cancer induction with the CT component contributing up to 81% of the total effective dose (Huang et al., 2009; Kaushik et al., 2013). Consequently, modification of the CT imaging parameters has been identified as a significant step to reducing dose to individual patients (Kumar et al., 2012; IAEA, 2013).
South Africa (SA), one of the world’s high-burden countries (HBCs) with TB epidemics and the fifth highest number with estimated prevalent (undiagnosed active TB) cases (Churchyard et al., 2014; WHO, 2016) is witnessing a gradual increase in the use of this imaging modality. However, this increasing use of PET/CT in the diagnosis, staging, and assessment of therapy response in infected patients raises the important consideration of the associated radiation dose. The knowledge of the dose is essential for clinicians and radiographers in checking standards of good practice as an aid to optimization of patient protection and also determining associated risks so that the diagnostic technique is properly justified (Wall, 1996).
The measure of the potential detriment from a radiographic procedure is best quantified by the radiation protection quantity, effective dose (ED). ED is not directly measured, but calculated based on equivalent doses to organs and the radiosensitivities of the organs (McCollough and Schueler, 2000; Mettler et al., 2008). Therefore, assessing the ED of the CT component of a whole-body PET/CT by experimenting with real subjects are not only dangerous but impossible, and the estimation from the product of the scanner-derived whole-body DLP (Dose-Length Product) value and a conversion factor often neglects regional differences when determining CTDIvol (Computed Tomography Dose Index) and the conversion factor from DLP to ED (Inoue et al., 2015). Monte Carlo simulation software has become one of the ways of proffering solutions to these problems.
The aims of this study were thus to: (i) quantify the effects of CT model and exposure protocols on the overall radiation effective dose to patients for commonly performed CT techniques in an 18F-FDG PET/CT examination; (ii) assess if the overall PET/CT dose resulting from the change in CT model and protocols are within acceptable values in literature; and (iii) analyze possible parameters affecting the radiation dose from the CT component. Specifically, comparisons were made between dosimetry results obtained using the CT Expo® dosimetry program (version 2.4) from specific CT study parameters during PET/CT acquisition with two different PET/CT systems. The data presented in this study will provide guidance on where efforts on dose reduction will need to be directed to fulfill the requirements of optimization and also serve as a reference for future work.
2 Materials and methods
2.1 PET/CT system and protocols
Two 16-slice PET/CT systems from different manufacturers namely the General Electric Healthcare (Discovery STE, 16) consisting of a PET scanner with bismuth germanate oxide (BGO) crystals detector and the Philips Medical Systems (Gemini TF 16) with a lutetium-yttrium oxyorthosilicate (LYSO) crystals detector based PET, denoted as systems I and II, were considered for this study. Standard patient preparation included at least 5 h fasting or longer and a serum glucose level of less than 10 mmol/L (180 mg/dL) before 18F-FDG injection. PET images were acquired one hour after intravenous 18F-FDG administration typically in three-dimensional mode because of scanner enhanced sensitivity, at 3 min per bed position after CT acquisition with the patient positioned so that the PET scan matches the same anatomic extent imaged during the CT acquisition.
The acquisition parameters of the CT protocols in this study (Tabs. 1 and 2) were based on what is routinely used for clinical patients in each facility, following retrospective review. Helical transmission CT is performed at a photon energy between tube voltages 120–140 kVp, tube current-time was varying by using the automatic exposure control (AEC) technique over the individual patient’s anatomy on the basis of a scout view and relative to the prescribed noise index value: 1) a low-dose scan in which the CT component serves as a fast transmission source for attenuation correction and anatomical localization in previously acquired diagnostic CT examinations or 2) a standard radiation dose scan with IV contrast given for attenuation correction and diagnostic purposes.
Protocols A and B, in each unit, are the low-dose CT scan most frequently performed for PET attenuation correction and anatomic localization. Protocols C, D and E were for diagnostic scans with D and E predominantly for contrast-enhanced studies and patients with larger body habitus wherein the tube current are maximized. The total duration of PET/CT examination was about 25 minutes except in the case of melanoma patients.
Standard clinically applied CT exposure parameters used on different PET/CT systems (system I).
Standard clinically applied CT exposure parameters used on different PET/CT systems (system II).
2.2 CT dosimetry
The estimation of organ and effective dose (ED) from CT by the CT-Expo® dosimetry program (version 2.4) was carried out base on the selection of characteristic CT model, LightSpeed 16, and the Brilliance 16 scanners stored in the software database for each system. The CT Expo dosimetry program, an MS Excel application written in Visual Basic permits dose calculations for four gender-specific mathematical phantoms, namely: ADAM, EVA, CHILD and BABY (Kramer et al., 1982). On the account of acquisition protocols presented in Tables 1 and 2 with a prescribed imaging range (identical for all patients as determined by the ADAM/EVA phantom) covering the entire torso from skull base to the pelvis, embodying common sites of infection such as the cervical, mediastinal, abdominal, and pelvic lymph nodes – (Fig. 1) the effective dose from the CT component was calculated applying the International Commission on Radiological Protection (ICRP) Publication 103 (ICRP, 2007) tissue weighting factors.
 |
Fig. 1 The ADAM and EVA phantoms of “CT-expo®” used for CT dosimetry calculations: the division corresponds to the anatomical length of scan region. |
2.3 Internal dosimetry
The average activity (A) of 18F-FDG administered for adults (male and female) was assumed to be 305 MBq (8.2 mCi) (Sathekge et al., 2014). This activity has an uptake time of 60 min with intravenous contrast given. Equivalent dose, DT to a tissue or organ T from the administered activity A of 18F-FDG was computed by means of dose coefficients provided by the ICRP Publication 106 (ICRP, 2008) for a variety of organs and tissues of the adult hermaphrodite MIRD (Medical Internal Radiation Dose) phantoms, using:
 (1)
(1)
Whole body effective dose contribution from 18F-FDG PET was then estimated as previously reported by (Brix et al., 2005; Huang et al., 2009) for organs and tissue equivalent doses DT modified by tissue weighting factors in ICRP publication 103 (ICRP, 2007) as follows:
 (2)
(2)
2.4 Statistical analysis
The total effective dose 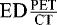 values resulting from each system and acquisition protocol were compared in terms of percentage differences calculated as described by equation (3) below:
values resulting from each system and acquisition protocol were compared in terms of percentage differences calculated as described by equation (3) below:
 (3)
(3)
The difference in the CT component contribution to the total dose between the two systems for all protocols was assessed with an unpaired t-test. A p-value below 0.05 was considered statistically significant.
3 Results
Organ and Tissue equivalent doses DT from administered 18F-FDG activity and their contribution (WT * DT) to the average PET scan effective dose 5.40 mSv are reported in Table 3. DT ranged from 2.38–39.65 mSv. Significant equivalent doses of 20.44, 11.59, 6.41 and 6.10 mSv were to the heart, brain, liver, and lungs respectively, due to their relatively higher metabolic activity and hence rapid blood supply, resulting in higher 18F-FDG uptake (Kaushik et al., 2013). The highest absorbed dose of 39.65 mSv to the bladder is attributed primarily to the final accumulation of the 18F-FDG tracer in the urine contained in the bladder, since the tracer is excreted by the kidney (Moran et al., 1999).
Figures 2A and 2B show effective dose values from the five study protocols. The second column in each figure represents total effective dose ED from the PET-CT examination, considering CT contribution with ICRP 103 tissue weighting factors. The total ED of the combined PET-CT scan for each system is summarized in Table 4. The percentage differences in total ED values between the two systems were 4.3–15% for the low-dose scan (A, B) and 4.1–11% for standard dose scans (C, D).
The CT effective dose contributions as seen in Figures 2A and 2B were comparable for both systems, with two sample t-test results showing no significant differences (p = 0.885; mean 9.55 mSv for system I vs. 10.3 mSv for system II). Statistical significance was defined as p-value < 0.05.
However, the slight differences observed in CT contribution resulted in higher total PET/CT dose for a specific system. For example, the ED from CT for low dose protocols A and B were 2.60 and 4.45 mSv for system I, whereas the value was 2.95 and 6.05 mSv for system II leading to variation of 12% and 30% between the two. Consecutively, the total PET/CT ED for system II was higher – 8.35 and 11.45 mSv, in contrast to 8.0 and 9.85 mSv for system I.
Organ and tissue equivalent doses DT from administered 18F-FDG activity and their contribution (WT * DT) to the average PET effective dose.
 |
Fig. 2 Mean effective dose values for systems I (A) and II (B), calculated by applying the ICRP 103 tissue weighting factors. |
Total PET/CT effective doses (mSv) with the percentage contributions of the CT and 18F-FDG-PET for systems I and II.
4 Discussion
In this study, the effect of CT model and protocols on total dose from a PET-CT procedure was evaluated. Computed tomography (CT) acquisition in PET-CT imaging is often performed for a variety of purposes, which includes diagnosis, anatomic localization and attenuation correction of the PET images (Alessio and Kinahan, 2012). However, there are possibilities of unnecessary exposure to high level of radiation dose, especially for CT imaging prescribed for diagnostic purposes.
The lower patient-specific effective dose 5.40 mSv from the administered average FDG activity of 305 MBq (8.2 mCi) in this study, compared to values of 6.25 mSv and 6.28 mSv previously reported by (Huang et al., 2009; Liu et al., 2016) respectively, is expected due to the greater FDG activities (328.77 and 370 MBq) from these studies. Analysis of the effective dose ED derived from the CT component for systems and protocols considered in this study reveals that while the ED was within the 25.0 mSv previously reported in the literature (Brix et al., 2005; Huang et al., 2009), ED values varied between 2.60–18.65 mSv for system I and 2.95–21.45 mSv for system II .
As such, the CT component of the examination contributed between (32–77%) for system I and (35–79%) for system II of the whole radiation dose. These are somewhat comparable with the 17–76% range from a review of CT protocols and CT dose contribution in PET/CT (Vandevoorde, 2011) and with a study by Mahmud et al. (2014), in which approximately 80% of the total PET/CT effective dose was attributable to the CT doses.
It is worth pointing out that, for each system, the relatively low dose (typically less than 6% of the total dose [Brix et al., 2005]) from the CT scout scan, to select the scan region and establish bed positions for PET acquisition, is not explicitly taken into account when considering the CT dose contribution.
The observed differences between the two systems could be attributed to factors related to scanner geometry and design (beam filtration, beam-shaping filter) and most importantly the user-adjustable factors (tube current and voltage, pitch factor, exposure time per rotation and slice collimation).
A short-geometry scanner following the inverse square law, radiation intensity varies with the inverse of the squared distance between radiation source and patient, will produce more dose to the patient than a long geometry scanner (Kalra et al., 2004). The total beam filtration (inherent + added) of varying material and thickness absorbs via photoelectric interactions the output photons at a function of their energy (Euclid Seeram, 2009; Brahme, 2014). These filters are noted for reducing radiation dose especially in the peripheral region of a field-of-view (FOV) (Kalra et al., 2004; Liu et al., 2013).
The most significant contributing factors to the disparities in estimated CT doses between the two systems from our study is perhaps the user-selectable parameters specifically the tube current-time product (mAs), pitch factor and collimation settings. Given the linear relationship between the tube current-time product (mAs) per rotation and the absorbed radiation dose (Coursey and Frush, 2008), the choice of mAs has a major effect on the radiation dose from any CT examinations.
The pitch (ratio of table feed per gantry rotation to the total collimated width of the X-ray beam) is inversely proportional to radiation dose if other scanning parameters are kept unchanged (Kalra et al., 2004; Coursey and Frush, 2008). Therefore, a higher pitch (faster table speed for a given collimation) will decrease radiation dose because of a shorter exposure time.
System I had a higher pitch factor compared to system II, which might explain its lower effective dose. The lower dose from protocols (C and D) in system II compared to system I, mean that the effect of an increase in the dose due to the lower pitch factor is slightly compensated by the use of the lower current-time product (mAs).
Additionally, overranging (unnecessary radiation exposure outside the planned scan length), an effect directly proportional to the pitch, beam collimation and reconstructed slice thickness is also a possible contributor to the observed difference in CT dose, given the dissimilarities in these technical parameters for the two systems (Tabs. 1 and 2).
The PET/CT effective doses from the two systems in this study are similar to previous measurements reported in the literature, with noticeable differences caused by the type of PET/CT scanner and protocol used in image acquisition. Brix et al. (2005) reported an effective dose of 14.1–18.6 mSv for diagnostic CT and 1.3–4.5 mSv for low-dose CT from PET/CT examinations performed in four German hospitals. Their study mostly includes 16-slice CT scanners and is generally comparable to our results. Huang et al. (2009) compared three different CT acquisition protocols using a humanoid (Alderson-Rando) phantom equipped with thermoluminescent dosimeters (TLD-100) and found the total PET/CT effective dose with a diagnostic CT protocol (per 370 MBq administered activity), to be in the order of 13.4–32 mSv. The CT dose component accounted for 54–81% of the combined dose, which is higher than systems I and II in this study.
Our estimated total PET/CT effective doses were also comparable with that published by Khamwan et al. (2010) and Kaushik et al. (2013), who reported values of (18.9 mSv) and (14.4 for females, 11.8 mSv for male patients) respectively. Mattsson and Söderberg (2011) also reported that the total effective dose from a PET study combined with a ʻlow-dose’ CT for attenuation correction and anatomical orientation is typically 14 mSv and 28 mSv with a diagnostic CT which are in good agreements with our findings for both systems.
Quinn et al. (2016) used patient-specific data to characterize the radiation dosimetry of two types of routine whole-body PET/CT protocols at their institution. They specifically evaluated the combined PET and CT scan dose for adult patients who undergo either the standard registration or full dose diagnostic CT techniques and found a mean total effective dose of 14 ± 1.3 mSv (11.0 to 17 mSv) and 24.4 ± 4.3 mSv (14.6 to 34.3 mSv) for all standard and diagnostic PET/CT patients respectively. The mean CT effective dose was 5.0 ± 1.0 mSv (2.9 to 7.2 mSv) and 15.4 ± 5.0 mSv (5.4 to 27.8 mSv) for the standard and diagnostic techniques. The results of this present study are well within this range.
These findings demonstrated that the radiation dose from a PET/CT scan depends on the PET/CT protocol, the patient’s size and physiology, the amount of injected activity and the make and model of the PET/CT scanner. The PET effective dose is modest and depends on the activity of the injected FDG (18F-Fluoro-deoxy-glucose) which is the same whether only a part of the body or the whole body is imaged. Major reductions in PET/CT dose are achieved from the CT component through the use of techniques such as automatic tube current modulation, iterative reconstruction, and adaptive filtering. Different vendors suggest different dose reduction methods; therefore every institution needs to develop scanner-specific protocols for implementing those methods.
The estimated dose presented in this study comes with some limitations. First, patient and organ dose calculations from CT with “CT-EXPO®” are based on anthropomorphic mathematical models for a standard person. These do not consider individual patients body sizes, organ positions and dimensions (Li et al., 2011). Different patient diameters, and the divergent location of relevant organs may cause different radiation absorption and hence some discrepancies in reported estimates. However, they are a reasonably good indicator for checking the relative compliance with reference dose values and for scan protocol optimization (Reiser et al., 2012). Accordingly, the use is justified for the purpose of our study as we did not intend to estimate doses for individual patients, rather evaluate the overall radiation dose resulting from CT protocol and model change. Second, dose estimation was based on mean tube current values, because CT-Expo® neglects the different approaches of automatic exposure control (AEC) methods operating with tube current modulation implemented on the CT component. Use of AEC systems is likely to lower patient dose, but the magnitude of such dose savings has no substantial effect on the effective dose values (Lechel et al., 2009). Finally, the dose coefficients used for internal absorbed dose assessment were based on numerous assumptions (Hays et al., 2002) that may result in variation from the “true” value.
5 Conclusion
The present study explored the effects of CT model and scan protocols on the overall dose from an 18F-FDG PET/CT procedure based on CT exam-specific parameters with the CT-Expo® dosimetry program. There is evidence of a slight variation in the effective dose contribution from the CT component for both PET/CT systems due to clinical technique differences and type of scanners. The presented dosimetric results also showed that radical changes to existing CT protocols are not necessary given that the total PET/CT dose from the two systems was typically within acceptable limits compared to current literature.
Though the present work is just one step in the direction of a complete ED estimate that uses the exposure settings of all X-ray pulses, CT protocol optimization measures and patient weight specific ED contributions, the observed variations in CT dose are however of concern, as the substantial part of the radiation exposure of PET/CT imaging is from the CT examination. The absence of a national diagnostic reference level (DRLs) to promote optimization in PET/CT imaging makes any decision regarding the need for optimization seems questionable. Further extended studies are needed to assess if a reduction in radiation exposure from the CT component while keeping the diagnostic quality at a clinically acceptable level to reduce the probability of stochastic effects is possible.
Acknowledgement
This work was funded by the authors from research allowance given to academic staff of the University of KwaZulu-Natal.
References
- Alessio AM, Kinahan PE. 2012. CT protocol selection in PET-CT imaging – image wisely. Virginia (United States): American College of Radiology. [Google Scholar]
- Alessio AM, Kinahan PE, Cheng PM, Vesselle H, Karp JS. 2004. PET/CT scanner instrumentation, challenges, and solutions. Radiol. Clin. North Am. 42: 1017–1032. [CrossRef] [PubMed] [Google Scholar]
- Brahme A. 2014. In: Comprehensive biomedical physics, Vol. 1, pp. 28–29. USA: Elsevier B.V. [Google Scholar]
- Brix G, Lechel U, Glatting G, Ziegler SI, Munzing W, Muller SP, Beyer T. 2005. Radiation exposure of patients undergoing whole body dual modality examinations. J. Nucl. Med. 46: 608–613. [PubMed] [Google Scholar]
- Churchyard GJ, Mametja LD, Mvusi L, Ndjeka N, Hesseling AC, Reid A, Babatunde S, Pillay Y. 2014. Tuberculosis control in South Africa: Successes, challenges, and recommendations. South Afr. Med. J. 104: 244–248. [CrossRef] [Google Scholar]
- Coursey CA, Frush DP. 2008. CT and radiation: What radiologists should know. Appl. Radiol. 37(3): 22–29. [Google Scholar]
- Euclid Seeram. 2009. Computed tomography physical principles, clinical applications, and quality control. 3rd edition. USA: Saunders. [Google Scholar]
- Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, Chung JK. 2000. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology 216: 117–121. [CrossRef] [PubMed] [Google Scholar]
- Hahm CR, Park HY, Jeon K, Um SW, Suh GY, Chung MP, Kim H, Kwon OJ, Koh WJ. 2010. Solitary pulmonary nodules caused by Mycobacterium tuberculosis and Mycobacterium avium complex. Lung 188: 25–31. [CrossRef] [PubMed] [Google Scholar]
- Hays MT, Watson EE, Thomas SR, Stabin M. 2002. MIRD dose estimate report No. 19: Radiation absorbed dose estimates from 18F-FDG. J. Nucl. Med. 43: 210–214. [PubMed] [Google Scholar]
- Huang B, Law MW, Khong PL. 2009. Whole-body PETCT scanning: Estimation of radiation dose and cancer risk. Radiology 251: 166–174. [CrossRef] [PubMed] [Google Scholar]
- IAEA. 2013. Radiation protection of patients (RPOP). https://rpop.iaea.org/RPOP/RPoP/Content/InformationFor/HealthProfessionals/index.htm. Accessed 11 April 2017. [Google Scholar]
- ICRP Publication 103. 2007. The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 37: 2–4. [Google Scholar]
- ICRP Publication 106. 2008. Radiation dose to patients from radiopharmaceuticals Addendum 3 to ICRP Publication 53. Ann. ICRP 38: 1–197. [CrossRef] [PubMed] [Google Scholar]
- Inoue Y, Nagahara K, Tanaka Y, Miyatake H, Hata H, Hara T. 2015. Methods of CT dose estimation in whole-body 18F-FDG PET/CT. J. Nucl. Med. 56: 695–700. [CrossRef] [PubMed] [Google Scholar]
- Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard JA, Saini S. 2004. Strategies for CT radiation dose optimization. Radiology 230: 619–628. [CrossRef] [PubMed] [Google Scholar]
- Karam M, Roberts-Klein S, Shet N, Chang J, Feustel P. 2008. Bilateral hilar foci on 18F-FDG PET scan in patients without lung cancer: variables associated with benign and malignant etiology. J. Nucl. Med. 49: 1429–1436. [CrossRef] [PubMed] [Google Scholar]
- Kaushik A, Jaimini A, Tripathi M, D’Souza M, Sharma R, Mishra AK, Mondal A, Dwarakanath BS. 2013. Estimation of patient dose in 18F-FDG and 18F-FDOPA PET/CT examinations. J. Cancer Res. Ther. 9: 477–483. [CrossRef] [PubMed] [Google Scholar]
- Khamwan K, Krisanachinda A, Pasawang P. 2010. The determination of patient dose from 18F-FDG PET/CT examination. Radiat. Prot. Dosim. 141(1): 50–55. [CrossRef] [Google Scholar]
- Kim IJ, Lee JS, Kim SJ, Kim YK, Jeong YJ, Jun S, Nam HY, Kim JS. 2008. Double-phase 18F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur. J. Nucl. Med. Mol. Imaging 35: 808–814. [CrossRef] [PubMed] [Google Scholar]
- Kramer R, Zankl M, Williams G, Drexler G. 1982. The calculation of dose from external photon exposures using reference human phantoms and Monte Carlo methods Part I. GSF-Report S-885. [Google Scholar]
- Kumar S, Pandey AK, Sharma P, Malhotra A, Kumar R. 2012. Optimization of the CT acquisition protocol to reduce patient dose without compromising the diagnostic quality for PET-CT: a phantom study. Nucl. Med. Commun. 33: 164–170. [CrossRef] [PubMed] [Google Scholar]
- Lechel U, Becker G, Langenfeld-Jäger G, Brix G. 2009. Dose reduction by automatic exposure control in multi-detector computed tomography: comparison between measurement and calculation. Eur. Radiol. 19: 1027–1034. [CrossRef] [PubMed] [Google Scholar]
- Li X et al. 2011. Patient-specific radiation dose and cancer risk estimation in CT: part II Application to patients. Med. Phys. 38: 408–420. [CrossRef] [PubMed] [Google Scholar]
- Liu D, Khong LP, Gao Y, Mahmood U, Quinn B, St.Germain J, Xu XG, Dauer LT. 2016. Radiation dosimetry of whole-body dual-tracer 18F-FDG and 11C-acetate PET/CT for hepatocellular carcinoma. J. Nucl. Med. 57: 907–912. [CrossRef] [PubMed] [Google Scholar]
- Liu F, Wang G, Cong W, Hsieh SS, Pelc NJ. 2013. Dynamic bowtie for fan-beam CT. J. X-ray Sci. Technol. 21(4): 579–590. [Google Scholar]
- Mahmud MH, Nordin AJ, Ahmad Saad FF, Fattah Azman AZ. 2014. Estimation of patient radiation dose from whole body 18FFDG PET/CT examination in cancer imaging: a preliminary study. J. Phys.: Conf. Ser. 546: 012008. [Google Scholar]
- Mattsson S, Söderberg M. 2011. Radiation dose management in CT, SPECT/CT and PET/CT techniques. Radiat. Prot. Dosim. 147(1–2): 13–21. [CrossRef] [Google Scholar]
- McCollough CH, Schueler BA. 2000. Calculation of effective dose. Med. Phys. 27: 828–837. [CrossRef] [PubMed] [Google Scholar]
- Mettler FA, Huda W, Yoshizumi TT, Mahesh M. 2008. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248: 254–263. [CrossRef] [PubMed] [Google Scholar]
- Moran JK, Lee HB, Blaufox MD. 1999. Optimization of urinary FDG excretion during PET imaging. J. Nucl. Med. 40: 1352–1357. [PubMed] [Google Scholar]
- Quinn B, Dauer Z, Pandit-Taskar N, Schoder H, Dauer LT. 2016. Radiation dosimetry of 18F-FDG PET/CT: incorporating exam-specific parameters in dose estimates. BMC Med. Imaging 16: 41. [CrossRef] [PubMed] [Google Scholar]
- Reiser MF, Hricak H, Knauth M. 2012. In: Radiation dose from Multidetector CT. 2nd edition, pp. 565–566. Berlin: Springer-Verlag. [Google Scholar]
- Sathekge MM, Vorster M, Stoltz A, Jacobs AG. 2014. Imaging of pulmonary tuberculosis with 18F-Fluoro-Deoxy-Glucose and 18F- Ethylcholine. Open Nucl. Med. J. 6: 16–17. [Google Scholar]
- Skoura E, Zumla A, Bomanji J. 2015. Imaging in tuberculosis. Int. J. Infect. Dis. 32: 87–93. [CrossRef] [PubMed] [Google Scholar]
- Soussan M, Brillet PY, Mekinian A, Khafagy A, Nicolas P, Vessieres A, Brauner M. 2012. Patterns of pulmonary tuberculosis on FDG-PET/CT. Eur. J. Radiol. 81: 2872–2876. [CrossRef] [PubMed] [Google Scholar]
- Vandevoorde C. 2011. CT protocols and CT dose contribution in PET/CT. Department of Medical Physics and Radiation Protection, Ghent University, Ghent, Belgium. [Google Scholar]
- Wall BF. 1996. How to assess the dose to the patient in diagnostic radiology. Chilton, Didcot (UK): National Radiation Protection Board NRPB. [Google Scholar]
- World Health Organization. 2016. Global Tuberculosis Report. http://www.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf. Accessed 06 April 2017. [Google Scholar]
Cite this article as: Adeleye B, Chetty N. 2018. Radiation dose from 18F-FDG PET/CT procedures: influence of specific CT model and protocols. Radioprotection 53(2): 107–113
All Tables
Standard clinically applied CT exposure parameters used on different PET/CT systems (system I).
Standard clinically applied CT exposure parameters used on different PET/CT systems (system II).
Organ and tissue equivalent doses DT from administered 18F-FDG activity and their contribution (WT * DT) to the average PET effective dose.
Total PET/CT effective doses (mSv) with the percentage contributions of the CT and 18F-FDG-PET for systems I and II.
All Figures
 |
Fig. 1 The ADAM and EVA phantoms of “CT-expo®” used for CT dosimetry calculations: the division corresponds to the anatomical length of scan region. |
| In the text | |
 |
Fig. 2 Mean effective dose values for systems I (A) and II (B), calculated by applying the ICRP 103 tissue weighting factors. |
| In the text | |
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.




